
Quick Links
Resources
About MyLeukemiaTeam
Powered By





Bispecific T-cell engager (BiTE) treatment and chimeric antigen receptor (CAR) T-cell therapy are two promising treatments for difficult-to-treat or relapsed blood cancers, including leukemias. Both treatments are types of immunotherapy, a category of treatment that uses the body’s immune system to better target tumor cells. However, there are differences in these drugs’ mechanisms, how they are created, and who is currently eligible to receive them.
This chart compares the major differences and similarities between BiTE treatment and CAR-T cell therapy treatment for leukemia.
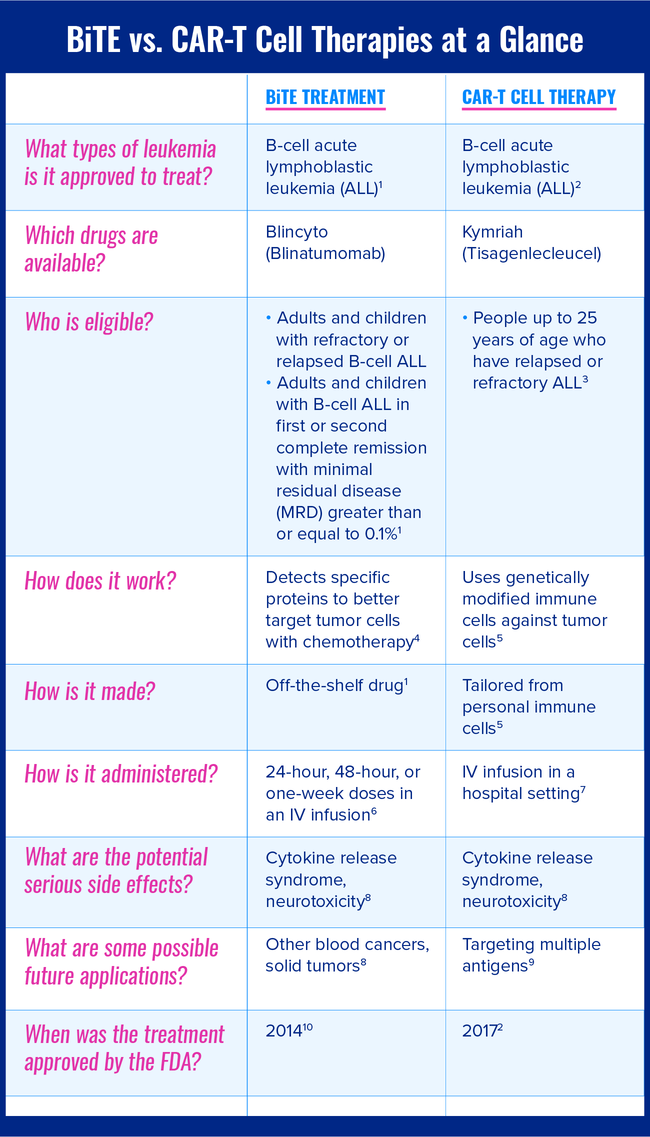
The following sections describe BiTE treatment and CAR-T cell therapy in greater detail.
Currently, only one form of BiTE treatment is available to treat leukemia. Blincyto (blinatumomab) is approved by the U.S. Food and Drug Administration (FDA) for people with refractory or relapsed B-cell acute lymphoblastic leukemia (ALL).
It is also approved for people who are in hematological remission from B-cell ALL, but still show minimal residual disease (MRD). Hematological remission means that the bone marrow contains an appropriate amount of white blood cells and that there are no signs or symptoms of leukemia. However, recently developed molecular assay testing allows the detection of MRD — tumor cells that would escape conventional microscope testing. These new tests are powerful enough to detect 1 cancer cell among 1 million normal cells. Eliminating MRD is important. Even if a person seems clinically to be in remission, persistent MRD is the strongest predictor of an eventual poor prognosis.
BiTE treatment works by inducing cytotoxic T cells to fight tumor cells. These drugs connect proteins in T cells (the immune cells that kill cancer and infected cells) to proteins in B cells (the malignant cells in B-cell ALL). In the case of Blincyto, the drug attaches itself to a protein called CD19, which exists on B cells. At the same time, it attaches to the CD3 protein on a T cell. When the T cell and the B cell are connected, the B cell is destroyed.

Blincyto is administered intravenously. Blincyto treatment begins in the hospital, so medical professionals can monitor you for adverse reactions. After three days, treatment either continues at home or under the supervision of an outpatient clinic or infusion center. Bags of Blincyto are administered through portable IV pumps. These bags can last 24 hours, 48 hours, or one week. Treatment generally lasts for four weeks, with a two-week pause before a second cycle of Blincyto, if needed.
Common side effects of Blincyto include fever, headache, and infection. Less common side effects of Blincyto include gastrointestinal symptoms, anemia, low blood pressure, and low appetite. Blincyto treatment can cause neurotoxicity — neurological symptoms that include tiredness and cognitive difficulties, such as slurred speech and disorientation. In rare cases, seizures can occur. About two-thirds of people taking Blincyto will experience some form of neurotoxic symptom.
Another serious side effect of Blincyto is cytokine release syndrome (CRS); 7 percent to 15 percent of people who are treated with Blincyto will experience CRS. CRS occurs when immune cells release too many cytokines (proteins that direct immune response), causing a massive inflammatory reaction. The effects of CRS can vary widely, from mild flu-like symptoms to organ failure. CRS can often be prevented or managed with Dexamethasone, a corticosteroid drug.
BiTE is a promising treatment for people with refractory B-cell ALL. In studies, Blincyto has shown itself to be more effective than standard chemotherapy in treating the disease, with people who receive Blincyto surviving longer and enjoying a better quality of life. Blincyto may also work as a “bridge” to other treatments, eliminating enough tumor cells in some people with advanced B-cell ALL to allow them to receive stem cell transplantation — in most cases an allogeneic stem cell transplant from donated hematopoietic stem cells. Scientists are also studying the effectiveness of experimental new BiTEs that target proteins other than CD19 in malignant leukemic cells.
One type of CAR-T cell treatment is currently available to treat leukemia. Kymriah (tisagenlecleucel) is currently approved to treat children and young adults with leukemia. Specifically, Kymriah is FDA-approved for people up to 25 years of age who have relapsed or refractory ALL.
Unlike BiTE treatment, which uses the same standard drug for every person treated, CAR-T cell therapy is created out of your own personal immune cells. T cells are taken from a person’s blood and genetically modified to contain chimeric antigen receptors (CARs). CARs are designed to bond with certain proteins on tumor cells, so the T cells can better target malignancies. The target antigen for Kymriah is the CD19 protein, which is found on B cells — the cells that become malignant in many types of leukemia and lymphoma, including B-cell ALL.

CAR-T cell activation therapy begins with a process called leukapheresis, which collects T cells in a process similar to donating blood. Usually, an IV is put in each arm. Whole blood is removed through one arm, filtered for T cells by a machine, and then returned to the body through the other arm. (Sometimes, a single catheter with two IV ports is used.) Leukapheresis takes a few hours.
The collected T cells are taken to a lab, where they are genetically modified to contain the appropriate CARs. This modification can take from less than a week to several weeks. Before receiving CAR-T cell therapy, people will usually have a few days of conditioning chemotherapy. This chemotherapy improves the body’s receptivity to the therapy. After chemotherapy is complete, the CAR-T cell infusion can be performed in a process similar to a blood transfusion. Once the infusion is over, the person who received the CAR-T cell treatment must stay in the hospital for a few days because of the risk of side effects.
Like BiTE treatment, CAR-T cell therapy can cause cytokine release syndrome and neurotoxicity. However, people receiving CAR-T cell therapy are more likely to develop CRS than people receiving BiTE treatment. A quarter to two-thirds of people undergoing CAR-T cell therapy will experience some level of CRS, and a smaller group will experience high-grade CRS, a potentially fatal condition which requires intensive care. After infusion, the risk period for side effects lasts two to three months, and people who undergo CAR-T cell therapy may need to be readmitted to the hospital to manage these side effects. Because of the serious risks, people who undergo this therapy must remain close to their treatment center afterward.
CAR-T cell therapy has shown impressive response rates against difficult-to-treat B-cell leukemias, with clinical trials showing remission rates of up to 94 percent. However, the side effects of CRS and neurotoxicity present potential obstacles. Because CAR-T cell therapy is especially effective against blood cancers, it is being investigated as a treatment for other types of leukemias and lymphomas, possibly as a first-line treatment. It is hoped that newer techniques for CAR-T cell therapy might result in fewer side effects. In addition, scientists are working on modifying CAR-T cell therapy so an “off-the-shelf” version is available. This might eliminate the need for each individual undergoing CAR-T cell therapy to have leukapheresis, as well as the time and cost of tailoring the therapy.
Get updates directly to your inbox.



Sign up for free!
Become a member to get even more




A MyLeukemiaTeam Member
Joann, prayers on the way. Hang in there. We are here for you.
We'd love to hear from you! Please share your name and email to post and read comments.
You'll also get the latest articles directly to your inbox.